Nuclear Magnetic Resonance Imaging (NMRI), better known as Magnetic Resonance Imaging (MRI) in medical parlance, is an invaluable tool in the study of the neurological system, soft tissue and musculo-skeletal system disorders. The word “Nuclear” was intentionally dropped later, as the procedure could then be wrongly interpreted by patients in relation to “ionizing radiation”, which certainly is not the case. However, the term Nuclear Magnetic Resonance (NMR) continues to be used in other (non-medical) fields of science, such as analytical chemistry, physics, biochemistry, petroleum industry, analysis of biological samples etc. In either case, the procedure and the basic principles remain the same. Paul Lauterbur was one of the pioneering inventors of this seemingly tough technological field.
Matter is made up of atoms, which in turn, are composed of negatively charged electrons orbiting around the nucleus (look at the animation of a
Helium atom on the left), consisting of positively charged protons and charge-less neutrons (with the exception of Hydrogen
1H nucleus, which contains a single proton and
no neutron). These subatomic particles (electron, proton etc) somehow, can not be understood in terms of shape or color; instead they are denoted by their charge, mass or spin (angular momentum). An even number of them will cancel each other’s spin [just like two revolving spheres, in touch with each other would, in a ‘classical world’ (if one rotated clockwise, the other would rotate anticlockwise, canceling any resultant spin)].
Hence, a net resultant spin would result in the nucleus only if it contained an odd (unpaired) number of protons, an odd number of neutron or both. [The concept that certain nuclear species had angular momentum was first suggested by Wolfgang Pauli, while explaining the fine structures in the Atomic spectra. In the presence of an external magnetic field, the spectral lines got split, depending on the strength of the field (
Zeeman Effect).]
Since nucleons bear a net charge (owing to the protons contained), the spinning nuclei will generate a magnetic field (since moving charges generate magnetic field). Each of these charged spinning ‘spheres’, hence, may be thought of as a tiny bar magnet having a magnetic dipole (that is a north-south orientation). [Electrons, similarly, have their own angular momentum though, responsible for molecular structure which nature uses, but they are not used by humans (
Milestones in Spin podcast)] When we talk about “MRI” in humans, we mean
proton nuclear magnetic resonance; i.e. NMR that detects the presence of hydrogen (proton) nuclei.
Our bodies have a plentiful of Hydrogen atoms: from the water within us, in cells and in extracellular fuid, (and to a lesser extent to the adipose tissue (fat)). These charge-carrying ‘unpaired’ protons (Hydrogen nuclei) rotate around their axes, but since all are spinning in a random fashion (as there’s no coordinator of any sorts); their net spin is zero, or in other words, their net magnetic moment is zero (as shown on the left).
Understanding spins aren't easy either. But, Prof. Stephen Hawking made it quite simpler for us using the real
classical world analogy of ‘playing cards’ in his famous book
A Brief History of Time
(
follow the link to learn more about ‘spin’). Having said that, the unpaired, positively charged protons having half integer (1/2) spins, behave like magnetic dipoles; it may now be understood easily that the spinning protons (nuclei) would align themselves to an externally applied magnetic field.
Thus, in a static magnetic field, the randomly oriented ‘tiny bar magnets’ align themselves up according to the applied magnetic field. These spinning protons (nuclei) also
precess (make an angle) with the applied magnetic field (Bo), much like a spinning top does when its angular momentum diminishes. An animation of a proton
precessing around a field is shown on the right.
[The magnet used for this purpose employs superconductivity. In a superconductive magnet, the electromagnet coils are immersed in liquid Helium at
minus 269 degree Centigrade. At such a low temperature, the coils loose ‘resistance’ to the flow of electrons, resulting in a highly stable and a very strong magnet. (However, any minute vibration in the superconducting magnet can lead to runaway Eddy current leading to a phenomenon called 'quenching', that happened in the
Large Hadron Collider 
at CERN, collapsing the whole setup.) Normally, 1.5 Tesla magnets are used, though nowadays 7 Tesla magnets have arrived. A 1 Tesla (1 Tesla=10,000 Gauss) magnet is 20,000 times stronger than the earth’s magnetic field)]. Also, note that we are considering magnetic moments
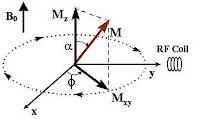
along the axis of the external field only, as far as the sum-total alignment of individual magnetic torque contributing to a 'macroscopic' magnetization (
M) is concerned. This is because the transverse components of the individual spins cancel out, as is seen in the 'cone' of the above picture. 600 persons of equal power, each pulling a rope either 30 degrees Northwest or 30 degree Northeast (in a 2 dimension), will certainly cancel out the 'east-west' vector, while the Northward vector will add-up. [It is this
M that produces the induction current in the receiver coil].
The protons have two choices. Either they have to align
parallel or
anti-parallel to the applied magnetic field (known as
spin-up and
spin-down position respectively). In any case, the protons only ‘
partially polarize’ since they tend to
‘make an angle’ with the applied static magnetic field. Spin down position is the higher energy state while spin-up state is the lower energy state of the spinning protons

(in the case of 23Na, there can be
4 spin-states instead of
2 as in 1H). (Obviously, a swimmer swimming upstream has more energy than his antiparallel counterpart.) The protons revolve (precess) around the direction of the magnetic field (Bo) at an angle, while at the same time they rotate around their own axis. Just as what happens in the solar system. [However, the upper (-1/2) and lower energy (+1/2) spin states are
almost equally populated with only a very small excess in the lower energy state at room temperature. Since, there are so many of them that we finally make some headway].
Let me clarify a bit. You've seen a spinning-top rotating around its own axis. Due to Earth’s gravitational field, the top ‘maintains’ an angle (with the perpendicular/vertical), more visible when its
angular momentum
(speed) decreases, as it continues spinning. The top may be seen to revolve around “the perpendicular” at an angle (=‘precess’), (in addition to its rotation around its “own axis”) during its course of revolution. [Watch the Video "
Introductory NMR & MRI Video 01 Precession and Resonance" to see what precession in NMR is]. This is what precession is about.
The frequency of precession is given by the Larmor relationship:
f=w/2*pi=yBo/2*pi (2*pi=360 degree)
w=angular freq. in radians per second; since there are 2*pi radians (360 degree) in a circle; we can find f, the frequency of rotation.
y is the magnetogyric (gyromagnetic) ratio, nuclear constant characteristic of every isotope. For 1H it is 42.5 MHz/T;
Bo=static magnetic field
The above equation is important, as we shall see later. Now let’s summarize what we learned so far.
Protons (nuclei) spin randomly in an atom. They tend to align with respect to an external magnetic field. These protons make an angle with the magnetic field as it goes about the magnetic field (while it also dutifully goes around itself), some parallel, and some antiparallel.
In MRI, our objective will be to
disturb this alignment of protons with a dose of radio frequency pulse, in a similar way I discussed in my
radio transmitter article but in a much, much bigger way. But since the ‘target’ (proton) is moving (precessing) around the field, we better ‘punched’ the target as if we were moving at the same angular velocity (so that the relative velocity was zero). Thus, when we apply the RF frequency pulses at the Larmor frequency, perpendicular to the magnetic field; the magnetic component (B1) of this electromagnetic wave temporarily knocks the protons out of alignment (see picture). If energy is absorbed by the nucleus, then the
angle of precession will change. Assuming the field strength to be 1 Tesla, the protons are revolving 42.5 million times per second; it is at this frequency we give the pulse (i.e. at the Larmor frequency).
The protons are pushed out of alignment and as the pulse ends, they ‘relax’ (more on how they ‘relax’, later) back to their undisturbed ‘equilibrium’ position. This causes emission of an RF signal (the Echo) that can be picked up by the receiver coil (the same transmitter coil that produced it, in most cases); a damped oscillating wave generated, as the ‘disturbed’ magnetic moments coming back to realign with the magnetic field. Now, the problem begins. We have applied a uniform/homogeneous magnetic field (Bo). There are a lot of protons but we don’t know who’s who and residing where. That is why we also apply
orthogonal magnetic field ‘gradients’
along the three (x, y, z) axes. [In a classroom, spray gradually ‘more’ yellow color in the front row and to the left than the back and to the right. In a similar way, spray blue color; hope your students don’t object. Now, every one of your students has a unique color: yellow, blue or green and with different hues]
Now that we get a decaying signal, which of high frequency; we mix it with a low frequency signal, in much the similar way as in
heterodyning, to produce an ‘interferogram’. This interference map is digitized, which is called the Free Induction Decay (FID). Thus, we do find too many frequencies in ‘the low frequency map’ which occur in ‘almost’ the same time. So, what can we do?
Waka Waka! In this football World Cup 2010 at South Africa, audience seems to have a deafening organ, what they call ‘
vuvuzela’. How are we going to analyze so many vuvuzelas when they are blowing at the ‘same time’? Just plot them in ‘frequency domain’ instead of ‘time domain’. Here’s Discrete
Fourier Transform (DFT) which will do happily for you. [Simply put, it samples the different frequencies and plots them; not all vuvuzelas have the same frequency]
Now, that fuzzy picture of multiple frequencies has a 'spatial information' (owing to its
orthogonal gradient magnetic field), contrast information (due to its ‘relaxation’ parameter), and foremost that it can be analyzed visually by humans, have enabled MRI to be a indispensable tool for the medical professional, as much as NMR has to the physicist or the discerning chemist. In MRI (NMR) it is not that important where or how energy is absorbed, but how quickly the excited protons revert back to its previous position is much more important, and hence the relevance of
T1 and T2 relaxation times.
By the way, contrast depends on the t1 and t2 relaxation, the surrounding chemical environment affecting relaxation, and of course the water content of the tissues [gray matter contains 10-15% more water than white matter.]
Finally, the article wouldn't be resourceful enough if I do not post some
MRI scans of the brain, this time, that of an epileptic patient (below).
(A sagittal section is obtained as the 'slice' takes a 'left to right' view (and vice versa); a coronal section means a 'front to back' view (or vice versa), and an axial slice means a virtual transverse section through the head.) Here's the picture of an actual MRI Machine below:
Naturally, the small tunnel may induce claustrophobia; the whirring acoustic noise from
switched gradient coils may be troublesome to the patient; any implanted pacemaker may be subjected to interference from the electrical field resulting in dislodgement or malfunction (as in other ferromagnetic objects such as wrist watch, key rings etc.). Moreover, sudden movement by the patient may induce voltage in semicircular canal producing vertigo, a sensation of giddiness. Advances in MRI technology is happening fast. Claustrophobia may now be ameliorated with a
wide bore MRI. A newly developed MRI scanner with Total Imaging Matrix (TIM) technology patients don't feel as claustrophobic, the imaging time is quick, quality of picture is better and even the acoustic noise is less (watch the
YouTube video here). Whatever be the shortcomings of MRI, the benefits far outweigh the risks and it is here to stay and evolve.
References:
Magnetic Resonance Imaging: David D. Stark, William. G. Bradley, Jr. NMR spectroscopy
Magnetic Resonance Imaging
MRI basics
Principles of NMR
NMR spectroscopy
Magnetic Resonance Imaging
MRI basics
Principles of NMR
William P. Dillon. Neuroimaging in Neurologic Disorders. In:
Harrison's Principles of Internal Medicine
, 17th Ed., Volume 2, McGraw Hill; 2008. p. 2491-2497.
Ian L. Pykett, Ph.D., Jeffrey H. Newhouse, M.D., Ferdinando S. Buonanno, M.D., Thomas J. Brady, M.D., Mark R. Goldman, M.D., J. Philip Kistler, M.D., & Gerald M. Pohost, M.D. (1982). Principles of Nuclear MagneticResonance Imaging Radiology
P.S. We will discuss
T1 and T2 relaxation,
fMRI,
tractography and NMR spectroscopy later.
Created: Jun 24, 2010; Last modified: Mar 10, 2014
 on healthy volunteers and found that they did impair memory and their functional coordinates could be reproducively mapped on fMRI scans (see figure on the left). I still shudder at the thought of what happened during my PG exam when I took a benzodiazepine.
on healthy volunteers and found that they did impair memory and their functional coordinates could be reproducively mapped on fMRI scans (see figure on the left). I still shudder at the thought of what happened during my PG exam when I took a benzodiazepine.