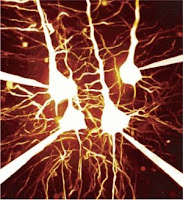
 Wouldn't it be nice if we mapped how the thought processes traveled across our brain, in real time? That's exactly what Mazahir Hasan et al of Max Planck Institute for Medical Research in Heidelberg, have enabled us to view, when an action potential (AP) is underway in the central nervous system (CNS). The researchers introduced fluorescent calcium indicator proteins (FCIP) into the brain cells of mice by means of viral gene vectors. Each time an AP was underway, a lot of ionic phenomena happened. For example, the fast Sodium channels (Na+) opened (letting positive charges to the interior of the cell) leading to depolarization, Potassium (K+) channels opened (to bring back the resting membrane potential to normal, since K+ egress out of the cells) and so on.
Wouldn't it be nice if we mapped how the thought processes traveled across our brain, in real time? That's exactly what Mazahir Hasan et al of Max Planck Institute for Medical Research in Heidelberg, have enabled us to view, when an action potential (AP) is underway in the central nervous system (CNS). The researchers introduced fluorescent calcium indicator proteins (FCIP) into the brain cells of mice by means of viral gene vectors. Each time an AP was underway, a lot of ionic phenomena happened. For example, the fast Sodium channels (Na+) opened (letting positive charges to the interior of the cell) leading to depolarization, Potassium (K+) channels opened (to bring back the resting membrane potential to normal, since K+ egress out of the cells) and so on.Next , the impulse is transmitted to the post-synaptic neuron through the agency of neurotransmitters. But, for this 'coupling' between the presynaptic and postsynaptic neurons to occur; Calcium ion (Ca++) levels in the synaptic knobs of the presynaptic neurons must rise for effective degranulation of the presynaptic vesicles. And that's precisely these researchers were banking upon.
Just before the degranulation of synaptic vesicles begins; calcium ion concentration surges. Such short calcium currents peak within milliseconds, making them the appropriate ions for studying fast neuronal activity. Previously scientists had measured such currents by using microelectrodes implanted within the brain; but this method was quite unsuitable in studying moving animals or for a longer time period. So, they went on to produce stable transgenic mouse lines responding to functional calcium indicators; (including 'inverse pericam' and 'camgaroo-2') using viral vectors. These transgenic mouse lines were under TET inducible promoter (tetracycline, a broad-spectrum antibiotic) control. The TET system offered the advantage of targeting combination of different neuronal cell assemblies. The other side of the Ptetbi (bidirectional promoter tetracycline) promoter was attached to the firefly luciferase gene. They were also sensitive to doxicline (another antibiotic belonging to the same category as tetracycline) in terms of regulation of luciferase, as well.
They then used a heteromeric sensor protein called D3cpv, which was made to produce in the nerve cells of the transgenic mice. Two subunits of this protein reacted to the binding of calcium ions in a way that when the yellow-fluorescent protein (YFP) lit up and the cyan-fluorescent protein (CFP) intensity diminished. When calcium was bound to the D3cpv complex; CFP (cyan fluorescent protein) and YFP (yellow fluorescent protein) came closer together bringing about FRET, in such a way that there was a visible color change, 'visually' or optically indicating the progression of action potential in real time. CFP and YFP are spectral variants of GFP linked together by a Ca++ sensitive linker.
They used 'two-photon imaging microscopy' to study this phenomenon. They excited thinned out rat skulls using two-photons simultaneously using 'mode-locked' Titanium-sapphire laser. They then amplified the signal using photomultipliers and analyzed them.
The resolution of the experiment was limited to less than 1 Hz (frequency of action potentials). They conferred that human thought processes might be mapped in much the same 'opto-physiologic way', in contrast to the usual electrophysiologic approach. Not only does the experiment throw light on the thought processes in real-time, but also, it is expected that it will be useful in the pathophysiology and treatment of Alzheimer's disease, Parkinson's disease and Huntington's chorea.
FCIP-positive cells were found in the hippocampal CA1 and CA3 regions, mossy fiber areas of the dentate gyrus, neocortical pyramidal cells and olfactory receptor neurons, they remarked. They studied cortical pyramidal cell, olfactory and optical responses in the mice in their experiment.
Last modified: never
Reference: Damian J Wallace, Stephan Meyer zum Alten Borgloh, Simone Astori, Ying Yang, Melanie Bausen, Sebastian Kügler, Amy E Palmer, Roger Y Tsien, Rolf Sprengel, Jason N D Kerr, Winfried Denk & Mazahir T Hasan. doi:10.1038/nmeth.1242
