Along with hypokinetic features, hyperkinetic features are also present (this is why PD also known as paralysis agitans; paralysis representing hypo- and agitans representing hyperkinetic features). They include tremor which is typically found at rest, and rigidity i.e. increased muscle tone of both flexor and extensor group of muscles. Rigidity is of lead-pipe type, meaning that you would feel uniform resistance, if you were to flex (or extend) patients arm passively, as in a malleable lead pipe. Cog wheel type rigidity is also seen, in which the limb moves in a series of catches as they are moved passively. Autonomic hyperactivity may be found in the form of sialorrhea (drooling of saliva). The person and his limbs assume a flexed posture, so that as the person moves, he is bent forward. The gait becomes festinant (festinating gait occurs as the man walks as if to catch up with his center of gravity, which now is tilted forward due to flexion). The patient gets demented (dementia) with time, and many other features develop.
It is not known what causes the destruction of dopaminergic neurons of the basal ganglia.
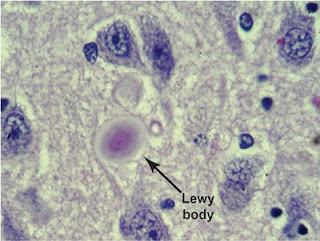 It was accidentally discovered that heroin addicts who were receiving dopes contaminated with a substance called MPTP, developed Parkinsonian like features. MPTP got converted to MPP+, a free radical, which destroyed the neurons. Now MPTP is used to create animal models of Parkinsonism for clinical research. In some familial types of the disease, 7 genes have been identified which when mutated lead to Parkinsonian features. For example, alpha synuclein (a gene product, a protein responsible for ‘marking’ proteins for removal called ubiquitination) and Parkin (another such protein product) combine and form what is called Lewy bodies. Intracellular Lewy bodies are constant features of Parkinsonism, but the roles they subserve are yet to be identified.
It was accidentally discovered that heroin addicts who were receiving dopes contaminated with a substance called MPTP, developed Parkinsonian like features. MPTP got converted to MPP+, a free radical, which destroyed the neurons. Now MPTP is used to create animal models of Parkinsonism for clinical research. In some familial types of the disease, 7 genes have been identified which when mutated lead to Parkinsonian features. For example, alpha synuclein (a gene product, a protein responsible for ‘marking’ proteins for removal called ubiquitination) and Parkin (another such protein product) combine and form what is called Lewy bodies. Intracellular Lewy bodies are constant features of Parkinsonism, but the roles they subserve are yet to be identified.Pharmacotherapy of Parkinsonism is far from satisfactory. We can not give the patient dopamine as such, because, it will get degraded before it reaches the brain. Moreover it can NOT reach the brain since to penetrate the lipid containing blood brain barrier, a drug has to be non polar (hydrophobic). So levodopa is used instead. It gets to the interior of the cells where it gets converted to dopamine(levodopa is thus called a prodrug). Carbidopa, a drug that prevents peripheral conversion of levodopa, is usually combined with levodopa and the action is synergistic. Drugs which prevent the degradation of dopamine by inhibiting its destroying enzymes are also used. They include the MAO-B inhibitor selegeline (deprenyl) (MAO for mono amine oxidase), COMT inhibitors tolcapone, entacapone etc (COMT for catechol O methyl transferase). Symptoms are alleviated with levodopa therapy but the effect wears off after 5-7 years and symptoms escalate. Selegeline, on the other hand, delays cell destruction. Cholinergic and dopaminergic systems in the brain strike a balance, actionwise. Thus anticholinergics such as benhexol or benztropine are also used. Dopaminergic drugs such as bromocryptine, lisuride, pergolide, cabergoline thus give some relief. (Conversely, antidopaminergic drugs like phenothiazine induce drug-induced Parkinsonism).
Therapy with antiapoptotic agents like desmethyl selegeline, antioxidant drugs, coenzyme Q10 are good therapeutic candidates. Infusion of glial cell line derived neurotrophic factors (GDNF) showed early promise. So did transplantation of fetal striatal tissue, the patients’ own carotid body or adrenal medullary tissues. DBS or deep brain stimulation, application of high frequency electric stimuli to specific brain tissues via subcutaneously located controls, also holds promise. Frank surgical means like pallidotomy (excision of globus pallidus interna) is rarely necessary.
A scientific approach and much research is direly needed to cure this neurodegenerative malady that is so prevalent among the aging population.
Last modified: never
Reference: hyper-links, unless specifically mentioned
No comments:
Post a Comment